Cefazolin Sodium Injection IP
Price 30 INR/ Vial
Cefazolin Sodium Injection IP Specification
- Origin of Medicine
- India
- Dosage Form
- Injection
- Physical Form
- Liquid
- Recommended For
- Hospital
- Dosage
- 500 mg/Vial
- Dosage Guidelines
- Prescription
- Suitable For
- Adults
- Storage Instructions
- Cool & Dry Place
Cefazolin Sodium Injection IP Trade Information
- Minimum Order Quantity
- 10 Vials
- Payment Terms
- Cash in Advance (CID)
- Supply Ability
- 500 Vials Per Month
- Delivery Time
- 7 Days
- Main Domestic Market
- All India
About Cefazolin Sodium Injection IP
Cefazolin Sodium Injection IP: Advantage & Applications
Cefazolin Sodium Injection IP is widely utilized across hospital settings due to its robust antibacterial properties. The plant application involves strict sterile manufacturing, ensuring consistent quality. Its advantage lies in rapid onset and efficient bacterial eradication, making it invaluable for post-surgical prophylaxis and acute infections. Usage spans from routine hospital procedures to critical care, underscoring its indispensability in frontline healthcare and emergency medicine.
Cefazolin Sodium Injection IP: Delivery, Supply & Sample Exchange
Cefazolin Sodium Injection IP is dispatched promptly with minimal expenditure, ensuring hospitals maintain seamless supply chains. Delivery times are streamlined, typically within standard pharmaceutical windows, and FOB port arrangements foster international exchange efficiency. Transparent sample policy allows healthcare establishments to evaluate product quality before commitment, enhancing trust and clinical outcomes. The entire supply process prioritizes reliability and responsive support for hospital procurement teams.
FAQs of Cefazolin Sodium Injection IP:
Q: How should Cefazolin Sodium Injection IP be administered for adult patients?
A: Cefazolin Sodium Injection IP is administered via intravenous injection as prescribed by a medical professional, ensuring appropriate dosage and technique for adult patients.Q: What is the recommended storage condition for this injection?
A: It is best stored in a cool, dry place to maintain its physical integrity and therapeutic potency until use.Q: When is the optimal time to use Cefazolin Sodium Injection IP in a hospital setting?
A: This medication is typically used during or after surgical procedures or for treating acute bacterial infections as directed by healthcare protocols.Q: Where is Cefazolin Sodium Injection IP manufactured and supplied from?
A: Cefazolin Sodium Injection IP has its origin in India and is supplied by reputable traders and suppliers within the region.Q: What is the process for obtaining samples of Cefazolin Sodium Injection IP?
A: Hospitals interested in evaluating the injection can request samples as per the providers sample policy, facilitating informed procurement decisions.Q: What are the key benefits of using Cefazolin Sodium Injection IP?
A: This injection offers efficient bacterial infection control, rapid onset of action, and reliability, contributing to improved patient outcomes in clinical settings.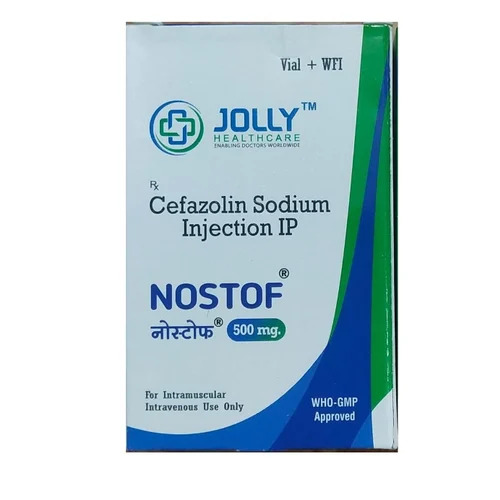

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Pharmaceutical Injection Category
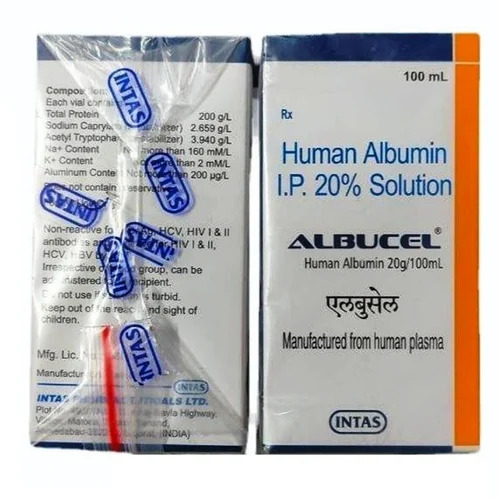
Human Albumin I.P. 20 Solution
Price 3750 INR / Bottle
Minimum Order Quantity : 1 Bottle
Dosage Guidelines : Prescription
Suitable For : Adults
Storage Instructions : Cool & Dry Place
Physical Form : Powder
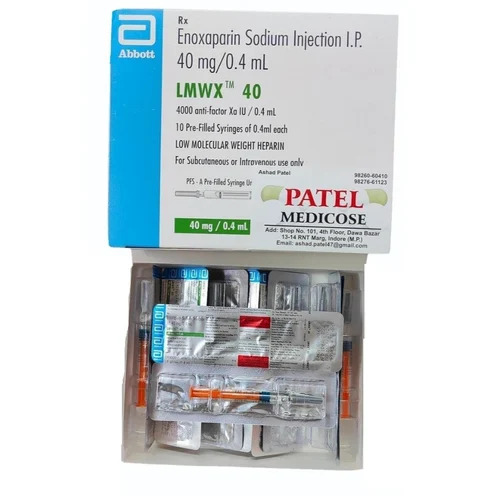
4ml Enoxaparin Sodium Injection I.P.
Price 145 INR / Piece
Minimum Order Quantity : 10 Pieces
Dosage Guidelines : Prescription
Suitable For : Adults
Storage Instructions : Cool & Dry Place
Physical Form : Liquid
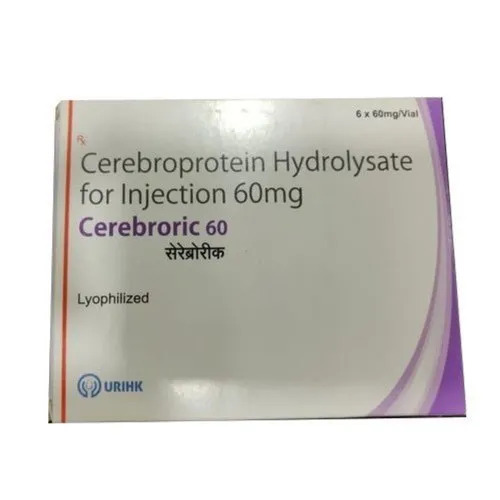
60 Cerebroprotein Hydrolysate Injection
Price 350 INR / Pack
Minimum Order Quantity : 10 Packs
Dosage Guidelines : Prescription
Suitable For : Adults
Storage Instructions : Cool & Dry Place
Physical Form : Liquid
50mg Liposomal Amphotericin B Injection
Price 1100 INR / Piece
Minimum Order Quantity : 10 Pieces
Dosage Guidelines : Prescription
Suitable For : Adults
Storage Instructions : Cool & Dry Place
Physical Form : Liquid
 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese